3. Discussion
Venoms
produced by snakes consist of many components, of which proteins and
peptides are the largest group. Many of these components have a
synergistic effect, which ensures the quick effect of venom on prey.
Victims hunted by a given snake species often belong to different
taxonomic groups, and have developed a variety of safeguards against
bites and its consequences. Therefore, the venom has agents acting
“universally” on a wide range of organisms, as well as those whose
activity is directed against a specific prey molecular targets [
5].
For each agent in the human body associated with hemostasis, we may find a homolog, activator or an inhibitor in the venom [6] operating on the principle of protein-protein interactions or enzymatic proteolysis [7]. Hemotoxic venoms, like the one produced by a common European adder,
affect blood vessel walls, platelets, coagulation, anticoagulation and
fibrinolysis. It often happens that, in the venom of a single species we
find components that are antagonists to hemostasis and even to
individual factors associated with it [
7].
Venom of
V. berus berus consists of approximately 25 proteins and peptides with enzymatic activity [
3,
4], and the total venom composition only of some Russian specimens has been described so far [
8]. Obtained gels of
V. berus berus
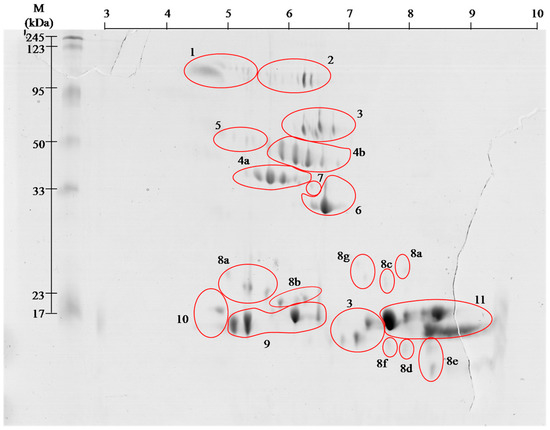
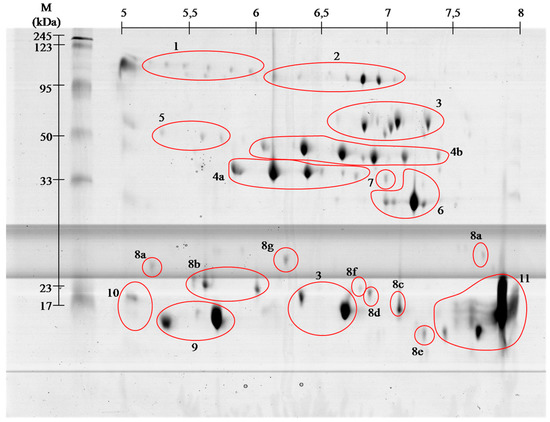
venom proteins contain even greater number of spots, but the
identification using MALDI ToF/ToF showed that they belong only to 11
families. With high probability it can be assumed that
the proteins in
this venom are highly post-translationally modified, as it is shown
clearly by visible spots trains in gels ( and ). This phenomenon is
characteristic for Viperidae family and was described already several times [
9,
10].
Our study indicates that the composition of the analyzed venom differs from that recently described in Latinović et al. [
8].
However, direct comparison of obtained results from those two studies
is not possible. Latinović et al. used normalized volumes of
corresponding 1-D gel protein bands and areas of elution peaks from
RP-HPLC for protein abundance estimation. On the other hand our study
employs protein quantity estimation method based on spots volume from
obtained 2-D gels after sample separation. Furthermore, it is not
possible to incorporate our peptidome results in to the protein chart
because of the method we used, as we have identified peptidome directly,
without prior separation. In this case, MALDI ToF/ToF technique does
not provide quantitative data,
so we cannot determine the content of
individual peptides in the venom. Keeping that in mind, direct
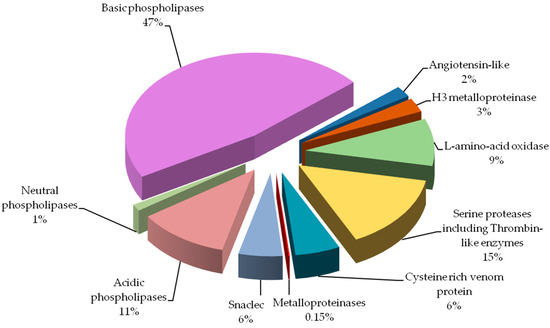
comparison of the percent shares of major protein groups would indicate
significantly
larger amount of phospholipases A2 (59% vs.
10%), and
much lower amount of serine proteinases (15% vs. 31%) in our
results. The most prominent difference observed would be
metalloproteinases share: 0.15% vs. 19%. Biological explanation for
observed discrepancies would be snake gender, Latinović et al. does not
declare it, and the snake habitat, in our case venom was obtained from
the snakes captured in natural environment in Slovak Republic, or age of
snakes and type of food. Influence of these factors was described
before and could attribute to the observed differences [
11].
Vipera berus berus venom has
mainly hemotoxic activity and identified proteins clearly meet the criteria for a wide range of hemotoxins [
3,
4].
Hemotoxins can be classified based on their effects on the following groups [6,7]: (i) activating blood coagulation factors;
(ii) anticoagulant agents;
(iii) inhibitors and activators of platelets;
(iv) agents affecting
fibrinolysis; and
(v) hemorrhagins.
Proteins of the first group affect
clotting factors or directly coagulate the fibrinogen (thrombin-like
enzymes) (spots # 4 and 5). Most of them, however, cause the formation
of fibrinopeptide A or B or, rarely both as it is in nature. Therefore,
created clots are unstable and prone to endogenous or venom-induced
fibrinolysis, which in turn leads to fibrinolysis syndrome
In the
anti-coagulant agents group, we include those components of the venom,
which inhibit tenas. There are mostly serine proteases (# 4 and 5),
protein C activators and phospholipases A2 (# 9–11).
Platelet
activating proteins cause thrombocytopenia and are predominantly C-type
lectin like proteins (# 8), and thrombin-like enzymes (# 4 and 5). In
turn, deactivation of platelets and following hemorrhage is caused by
disintegrins and snake venom metalloproteases (SVMPs) (# 2 and 7).
The
group of proteins responsible for fibrinolysis includes protein directly
capable of disrupting the fibrin (# 4 and 5) or plasmin activators.
The
last group of proteins is th
e hemorrhagins–cytolysins damaging blood
vessels and causing hemorrhages. They mostly include
metalloproteases (#
2 and 7) [
4,
5].
We found all the above-described groups of proteins in the venom of Vipera berus berus. The specificity of these proteins clearly explains hemotoxic properties of this venom.
Most
diverse group of proteins in European adder venom is the
snaclec
proteins belonging to C-type lectin like proteins. Most snaclec type
proteins are non-enzymatic homodimers of a weight 26 and 28 kDa composed
of subunits with weight about 13 and 18 kDa, and are responsible for
the
erythrocytes agglutination. They may also take the form of
heterodimers or oligomers, and contribute to the activation or
inhibition of human platelets [
12]. Performed separation under denaturing conditions confirms their monomeric weight in the range of 15 to 25 kDa ( and ). In the
V. berus berus venom we identified eight homologues of these proteins from different species of
Viperidae (# 8a–8g), constituting 5.5% of venom proteins and this is the first finding of these proteins in this species.
The largest group of proteins identified in the adder venom is a family of
phospholipases A2 (PLA
2) (60%). They are small enzymes with a mass of approximately 14 kDa corresponding to about 115–133 amino acid residues [
13].
Depending on the amino acid composition they are divided into acidic,
basic and neutral–all three groups we have identified in European adder
(# 9–11). The snake venom’s phospholipases of group I and II are widely
distributed in many snake species and are important neuro- and miotoxic
agents, causing the immobilization of the prey. Often in the venom of
snakes different types of phospholipases are present, which cause
different pharmacological effects starting with blood coagulation
disorders, through the inhibition of platelet aggregation, to blocking
of neuromuscular signaling and skeletal muscle paralysis [
14]. On gels ( and )
the areas with these proteins appear in the 15 kDa region throughout
the full used pH range, wherein the acidic, basic and neutral
phospholipase were identified, confirming the literature data [
13,
14].
Serine
proteases, also known as thrombin-like enzymes, are another big
identified group (15%, # 4 and 5). They constitute a collection of
enzymes that catalyze reactions involving a wide range of the blood
coagulation cascade, fibrinolysis and platelet aggregation. Specific
serine proteases catalyze usually only one or a few of the many
reactions involved in blood coagulation. They have the ability to cut
fibrinogen in the way similar to thrombin. This results in
clot
formation, acting not only through participation in the coagulation
pathway, but also b
y direct platelet aggregation [
15].
The molecular weight of these enzymes ranges from about 30 to 60 kDa.
On the obtained polyacrylamide gels serine proteases are located in the
area of pH 5–8 and the weight range of 35–50 kDa ( and ).
From the pharmacological point of view this group of proteins is very
interesting and promising since
they could be used in
hyperfibrinogenemia treatment, an important risk factor for ischemic
stroke and peripheral artery diseases [
16].
In the venom of the
Viperidae
family all classes of SVMPs (snake venom metalloproteinases) are
present, playing an important role in immobilizing prey by blocking the
transmission of nerve signals, and tissue proteolysis in the initial
digestion. They play an important role in the impairment of blood
clotting causing immediate local bleeding and delayed internal bleeding [
17,
18,
19].
As it is apparent from this research (# 2 and # 7)
SVMPs type III
containing metalloproteinase, disintegrin-like and a cysteine-rich
domains are the largest class of metalloproteinases in
V. berus berus
venom. However, this class has a very small share in the venom
proteome, less than 0.5%. Interestingly, earlier studies indicate a much
larger share of this group of proteins in the venom of
V. berus berus [
8].
In
the upper part of the gel a group of proteins identified as
Angiotensin-like peptide 2 (# 1) was found. The molecular weight of this
peptide is about 1 kDa, and the spot which contain proteins with this
short sequence on the basis of which the identification was made, have
weight almost one hundred times greater. This probably means that in the
venom of European adder there is so far undescribed protein that is
vasoactive, i.e.,
has a constricting or dilating effect on the caliber
of blood vessels [
20].
A second possibility is that the series of spots visible on gels (# 1)
contains the precursors of bioactive peptides. Due to the fact that not
all peptides were identified, there is a chance that considered venom
includes peptides having angiotensin-like properties. However, this
result requires more research as only one short peptide belonging to
this protein was identified.
Besides basic
phospholipase only two other proteins were assied to the database
entries as coming from the examined species. These include
l-amino acid oxidases (# 3) and
venom cysteine-rich proteins (# 6).
l-amino
acid oxidases are present in venoms of many snakes in large quantities
and their toxicity is primarily due to oxidative stress induced by H
2O
2, which is produced in enzymatic reaction of oxidative deamination of
l-amino acids [
21].
These proteins have a very wide range of action from anticoagulation
and inhibition of platelet aggregation to anti-viral and anti-bacterial
properties [
22,
23,
24,
25]. In obtained gels
l-amino acid oxidases appear in two areas ( and , # 3), and represent 9% of venom proteins. Spots in the upper molecular weight range correspond to the literature data [
25]
with weight of approximately 50 kDa. In turn, the spot in the lower
molecular weight region of the gel match only the data from the UniProt
(P0C2D7 (OXLA_VIPBB)) suggesting that this 88-amino acid protein has a
mass of approximately 10 kDa. This observed difference may be due to the
level of protein glycosylation, as in other species, or occurrence of
isoforms of this enzyme [
24,
26].
The second protein derived from
V. berus berus
is a member of
cysteine-rich venom protein CRISP (# 6). Proteins from
this group shows wide variety of biological activities. There are many
reports indicating that several venom-derived CRISPs could exhibit
neurotoxicity due to their inhibitory effect on different types of
ion
channels [
27,
28]. Our experiment showed that this is the 4th largest group of proteins in the analyzed venom (6%).
The
only protein that was
not found as a result of our experiment was
hyaluronidase. Hyaluronidase causes degradation of hyaluronic acid which
increases the permeability of the tissue at the bite site, and hence
the degree of absorption of the venom. Their action results in local
swelling, blistering and necrosis [
3].
Although numerous literature sources indicate that the venom has such
properties, the factor responsible for them has not yet been found.
Interestingly, despite many citations [
25,
29,
30,
31] only one work actually states the
presence of agents capable of carrying out the depolymerization of hyaluronic acid [
32]
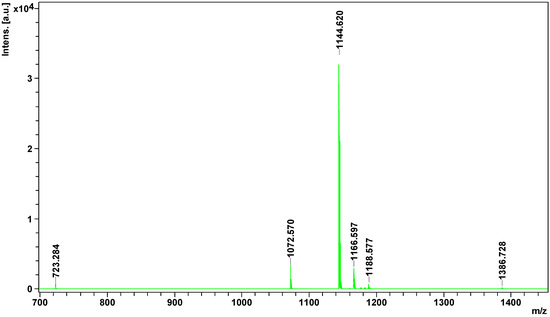
Peptidome
analysis showed the presence of 8 peptides in European adder venom, of
which only four could be identified.
All of them were identified as
bradykinin -potentiating peptides. Hence, all of them could be
inhibitors
of angiotensin-converting enzyme and would enhance the action of
bradykinin, and consequently act as hypotensive agents [
33]. Potentially, they could act just like captopril, an oral medication based on the peptide from
Bothrops jararaca venom. Interestingly, only one peptide detected in this experiment (1166.5968
m/
z) was previously identified in other
Vipera species [
34], others are of Crotalinae origins ().
Peptides contained in the venom have great pharmacological potential.
They are
poorly immunogenic and have evolutionary conserved tertiary
structure, obtained mostly by disulfide bonds and posttranslational
modifications [
35]. The most common of these modifications is pyroglutamate residue at the
N-terminus [
33,
34] observed in two peptides of
V. berus berus ().
It
is necessary to note that the meaningful identification for 2 out of 4
isolated peptides have been obtained only when the posttranslational
modification of deamination NQ was included in the Mascot search
parameters. Unfortunately, it is not possible to determine with our
current experimental setup if such a modification is of a natural origin
or it is an artifact.
Presented results
clearly show that the venom of European adder has mainly hemotoxic
effect, as inferred from a large number of proteins from the family of
metalloproteinases, serine proteases, L-amino acid oxidases or C-type
lectin-like proteins. They exhibit toxic effects on the vascular system,
causing abnormal blood clotting. Furthermore, l-amino
acid oxidases act by causing neuromuscular blockade, and lead to the
destruction of the cells by breaking cell membrane during its
depolarization. In the venom of this snake we also observe a few
proteins responsible for neurotoxicity, these are a cysteine-rich
proteins responsible for the blockade of nerve conduction and
phospholipases A2 possessing both neuro-, myo-, cyto- and
hemotoxic properties. Literature data indicate that the effects of
European adder venom is based mainly on the disorder of homeostasis and
the impairment of blood clotting process, as shown by the presented
results. This work describes for the first time the peptidome of V. berus berus.
Identified peptides potentially have blood pressure lowering properties
and may present a valuable target for further pharmacological
investigations.




Inga kommentarer:
Skicka en kommentar