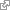- Olen kertaamassa RAAS- järjestelmää ja siinä on neprilysiinillä (NEP) myös osa.
- ACE2 ja neprilysiini kehkeyttävät angiotensiiniä Ang -(1-7) . ACE2 tekee sitä muodosta Ang-(1-8), joka on Angiotensiini-II. Neprilysiini muodostaa sitä Ang-(1-10).stä, joka on Angiotensiini-I. Ang-(1-7) toimii renoprotektiivisesti ja kardioprotektiivisesti ja käyttää GPCR-MAS aktivaatiotietä, joka tie opponoi ja vaimentaa ACE- Angiotensiini II- välitteisen verenpaineen säätelytien monipuolisia reseptorivaikutuksia ja täten molemmat tiet osallistuvat verenpaineen säätelynhomeostaasiin.
-
- MME-geenin koodama neprilysiini (NEP) on tyypin II transmembraaniglykoproteiini ja se tunnetaan myös tavallisena akuutin leukemian antigeenina (CALLA) tärkeänä solupintamerkitsijänä diagnosoitaessa akuuttia lymfaattista leukemiaa. koodautunutta proteiinia on pre-B-fenotyyppisissä leukemisissa soluissa, joita on 85% ALL-solusita. Proteiini ei ole kutienkaan rajoittunut leukemisiin soluihin vaan sitä löytyy useissa normaalikudoksissa. proteiini on neutraali endopeptidaasi, joka pilkkoo peptidejä hydrofobisten aminohappojen aminopuoellta ja pystyy inaktivoimaan useita peptidihormoneja8 glukagonia, enkefaliineja, P-substanssia, neurotensiiniä, oksytosiinia, bradykiniiniä (BK). Geeniä ilmenee duodenumissa, munuaisessa ja 8 muussa kudoksessa. Neprilysiinillä on vaihtoehtoisia nimiä monta, mutta suositeltu nimi on neprilysiini(NEP). Se tunnetaan myös mm. atriolysiininä, yleisenä akuuttin lymfosyyttileukemian antigeenina (CALLA)
- kalvon metallo-endopeptidaasina (MME), neutraalina endopeptidaasina (NEP), enkefalinaasina, CD 10 (Cluster of Differentiation 10), iho-fibriblastielastaasina (SFE) ja muitakin nimiä on ( kts. alla)
- PubMed:
- Official
Symbol MME
- membrane metalloendopeptidase
- Also known as
- NEP; SFE; CD10; CALLA; CMT2T; SCA43
- Summary
- The protein encoded by this gene is a type II transmembrane
glycoprotein and a common acute lymphocytic leukemia antigen that is an
important cell surface marker in the diagnosis of human acute
lymphocytic leukemia (ALL). The encoded protein is present on leukemic
cells of pre-B phenotype, which represent 85% of cases of ALL. This
protein is not restricted to leukemic cells, however, and is found on a
variety of normal tissues. The protein is a neutral endopeptidase that
cleaves peptides at the amino side of hydrophobic residues and
inactivates several peptide hormones including glucagon, enkephalins,
substance P, neurotensin, oxytocin, and bradykinin. [provided by RefSeq,
Aug 2017]
- Expression
- Biased expression in duodenum (RPKM 88.5), kidney (RPKM 83.7) and 8 other tissues See more
- Preferred Names
- neprilysin
- Names
- atriopeptidase
- common acute lymphocytic leukemia antigen
- membrane metallo-endopeptidase (neutral endopeptidase, enkephalinase, CALLA, CD10)
- membrane metallo-endopeptidase variant 1
- membrane metallo-endopeptidase variant 2
- neprilysin-390
- neprilysin-411
- neutral endopeptidase 24.11
- skin fibroblast elastase
Neprilysiiniin liittyvää lisätietoa PubMed artikkeleista.
-
Discovery of a CD10-negative B-progenitor in human fetal life identifies unique ontogeny-related developmental programs.
O'Byrne S, et al. Blood, 2019 Sep 26. PMID 31383639
-
Tumorous CD10 Is More Strongly Related to the Progression of Urothelial Carcinoma than Stromal CD10.
Kumagai-Togashi A, et al. Anticancer Res, 2019 Feb. PMID 30711939
-
Characterising the phenotype and mode of inheritance of patients with inherited peripheral neuropathies carrying MME mutations.
Lupo V, et al. J Med Genet, 2018 Dec. PMID 30415211
-
High expression of CD10 in anaplastic thyroid carcinomas.
Nakazawa T, et al. Histopathology, 2018 Sep. PMID 29791034
-
CD10 inhibits cell motility but expression is associated with advanced stage disease in colorectal cancer.
Raposo TP, et al. Exp Mol Pathol, 2018 Jun. PMID 29653092
See citations in PubMed for homologs of this gene provided by HomoloGe
- GeneRIF
-
Almost
one-third of fetal B-progenitors are CD10-ve PreProB-progenitors,
whereas, by contrast, PreProB-progenitors are almost undetectable (0.53%
+/- 0.24%) in adult Bone Marrow.
-
our
findings confirm that MME does represent the most common causative gene
responsible for late-onset AR-CMT2 in our population, but they do not
provide support for the hypothesis that heterozygous mutations in MME
are a direct cause of CMT.
-
receptor signaling and NEP are involved in the resistance of CNP to human mesangial proliferation and Col-IV expression.
-
Urinary
ACE2, neprilysin, and ADAM17 are increased in patients with diabetes
and could be used as early biomarkers to predict the incidence or
progression of CKD at early stages among individuals with type 2
diabetes. Abstract
Angiotensin converting enzyme 2 (ACE2) and neprilysin
(NEP) are metalloproteases that are highly expressed in the renal
proximal tubules. ACE2 and NEP generate renoprotective angiotensin (1-7)
from angiotensin II and angiotensin I, respectively, and therefore
could have a major role in chronic kidney disease (CKD). Recent data
demonstrated increased urinary ACE2 in patients with diabetes with CKD
and kidney transplants. We tested the hypothesis that urinary ACE2, NEP,
and a disintegrin and metalloproteinase 17 (ADAM17) are increased and
could be risk predictors of CKD in patients with diabetes. ACE2, NEP,
and ADAM17 were investigated in 20 nondiabetics (ND) and 40 patients
with diabetes with normoalbuminuria (Dnormo), microalbuminuria (Dmicro),
and macroalbuminuria (Dmacro) using ELISA, Western blot, and
fluorogenic and mass spectrometric-based enzyme assays. Logistic
regression model was applied to predict the risk prediction. Receiver
operating characteristic curves were drawn, and prediction accuracies
were calculated to explore the effectiveness of ACE2 and NEP in
predicting diabetes and CKD. Results demonstrated that there is no
evidence of urinary ACE2 and ADAM17 in ND subjects, but both enzymes
were increased in patients with diabetes, including Dnormo. Although
there was no detectable plasma ACE2 activity, there was evidence of
urinary and plasma NEP in all the subjects, and urinary NEP was
significantly increased in Dmicro patients. NEP and ACE2 showed
significant correlations with metabolic and renal characteristics. In
summary, urinary ACE2, NEP, and ADAM17 are increased in patients with
diabetes and could be used as early biomarkers to predict the incidence
or progression of CKD at early stages among individuals with type 2
diabetes.
-
Our
detailed analyses, with a substantial number of brain samples, provide
the first convincing evidence that DNA methylation of the NEP promoter
is not involved in Alzheimer disease development and progression.
-
Tumorous
CD10 is more strongly related to progression of urothelial carcinoma
than stromal CD10 and is an independent factor for UC prognosis
-
showed CD10 positivity in fibroblast-like stromal cells and fibrous material
-
these
data indicated under cigarette smoke condensate treatment; losing of
membrane p120ctn could upregulate surface NEP protein level and thus
facilitate BEAS-2B cell migration.
-
High expression of CD10 is associated with lymph node invasion in colorectal cancer.
-
We
observed an increase of VCAM-1 urine levels in diabetic
nephropathy-basal patients compared to diabetic controls and an increase
of urinary neprilysin in DN-treated patients with persistent
albuminuria
- Tietoja Neprilysiiniproteiinista
- https://www.ncbi.nlm.nih.gov/protein/NP_000893.2
- Tässä on isoformi a (1..750 amiohappoa) TM kohta merkattutummalla (29..51) ,
- Aktiivikohta merkattu isoilla kirjaimilla. 543N, 544A, 581V, 584H, 585E, 588H, 647E, 690F, 691A 712H, 718R.
- Zinkkiä sitova kohta m, kolme aminohappoa osuu aktiiviin kohtaan, tummalla merkatut.
- Näkyy olevan yksi motiivi llxxl (674-680) (lie tumareseptori?)
- peptidista koko mitalta 79-748 on M12 peptidaasiperheen M12 endoteliinia konvertoiva entsyymi I (ECE-1) .
- N-terminaalinen sytoplasminen osa:
1 mgksesqmdi tdintpkpkk kqrwtple
ORIGIN
1 mgksesqmdi tdintpkpkk kqrwtpleis lsvlvlllti iavtmialya tyddgickss
61 dciksaarli qnmdattepc tdffkyacgg wlkrnvipet ssrygnfdil rdelevvlkd
121 vlqepktedi vavqkakaly rscinesaid srggepllkl lpdiygwpva tenweqkyga
181 swtaekaiaq lnskygkkvl inlfvgtddk nsvnhvihid qprlglpsrd yyectgiyke
241 actayvdfmi svarlirqee rlpidenqla lemnkvmele keianatakp edrndpmlly
301 nkmtlaqiqn nfsleingkp fswlnftnei mstvnisitn eedvvvyape yltklkpilt
361 kysardlqnl mswrfimdlv sslsrtykes rnafrkalyg ttsetatwrr canyvngnme
421 navgrlyvea afageskhvv edliaqirev fiqtlddltw mdaetkkrae ekalaikeri
481 gypddivsnd nklnneylel nykedeyfen iiqnlkfsqs kqlkklrekv dkdewisgaa
541 vvNAfyssgr nqivfpagil qppffsaqqs nslnyggigm VigHEitHgf ddngrnfnkd
601 gdlvdwwtqq sasnfkeqsq cmvyqygnfs wdlaggqhln gintlgEnia dngglgqayr
661 ayqnyikkng eekllpgldl nhkqlfflnF Aqvwcgtyrp eyavnsiktd vHspgnfRii
721 gtlqnsaefs eafhcrknsy mnpekkcrvw
//
Tietoja Neprilysiinin perheestä "M13".
Metallopeptidase family M13 includes neprilysin (NEP) and other members:
M13
family of metallopeptidases includes
neprilysin (neutral endopeptidase,
NEP, enkephalinase, CD10, CALLA, EC 3.4.24.11),
endothelin-converting
enzyme I (ECE-1, EC 3.4.24.71),
erythrocyte surface antigen KELL
(ECE-3),
phosphate-regulating gene on the X chromosome (PHEX),
soluble
secreted endopeptidase (SEP), and
damage-induced neuronal endopeptidase
(DINE)/X-converting enzyme (XCE).
These proteins consist of a
short
N-terminal cytoplasmic domain,
a single transmembrane helix,
and a
larger C-terminal
extracellular domain containing the
active site.
Proteins in this family fulfill a broad range of physiological roles due
to the greater variation in the S2' subsite allowing substrate
specificity.
NEP is expressed in a variety of tissues including kidney
and brain, and is involved in many physiological and pathological
processes, including blood pressure and inflammatory response. It
degrades a wide array of substrates such as substance P (SP), enkephalins,
cholecystokinin (CCK), neurotensin and somatostatin. It is an important
enzyme in the regulation of amyloid-beta (Abeta) protein that forms
amyloid plaques that are associated with Alzeimers disease (AD).
ECE-1
catalyzes the final rate-limiting step in the biosynthesis of
endothelins via post-translational conversion of the biologically
inactive big endothelins. Like NEP, it also hydrolyses
bradykinin,
substance P, neurotensin and Abeta.
Endothelin-1 overproduction has
been implicated in various diseases, including stroke, asthma,
hypertension, and cardiac and renal failure.
Kell is a homolog of NEP
and constitutes a major antigen on human erythrocytes; it preferentially
cleaves big endothelin-3 to produce bioactive endothelin-3, but is also
known to cleave substance P and neurokinin A.
PHEX forms a complex
interaction with fibroblast growth factor 23 (FGF23) and matrix
extracellular phosphoglycoprotein, causing
bone mineralization. A
loss-of-function mutation in PHEX disrupts this interaction leading to
hypophosphatemic rickets; X-linked hypophosphatemic (XLH) rickets is the
most common form of metabolic rickets.
ECEL1 is a
brain metalloprotease
involved in the critical role in
the nervous regulation of the
respiratory system, while
DINE (damage induced neuronal endopeptidase)
is abundantly expressed in the hypothalamus and its expression responds
to nerve injury as well.
Thus, majority of these
M13 proteases are prime
therapeutic targets for selective inhibition.
https://www.ncbi.nlm.nih.gov/pubmed
Liekö yhteyttä ylläolevilla tekijöillä respiraation kerebraaliseen säätelyyn? Katsotaan,mitä tiede löytää lähi tulevaisuudesa.
Respiratory
regulation & interactions with neuro-cognitive circuitry.
It is increasingly being recognized that active
control
of breathing - a key aspect of ancient Vedic meditative practices, can
relieve stress and anxiety and improve cognition. However,
the
underlying mechanisms of respiratory modulation of neurophysiology are
just beginning to be elucidated. Research shows that brainstem circuits
involved in the motor
control of
respiration receive input from and can directly modulate activity in subcortical circuits, affecting emotion and arousal. Meanwhile,
brain regions involved in the sensory aspects of
respiration, such as the olfactory bulb, are like-wise linked with wide-spread
brain
oscillations; and perturbing olfactory bulb activity can significantly
affect both mood and cognition. Thus, via both motor and sensory
pathways, there are clear mechanisms by which
brain
activity is entrained to the respiratory cycle. Here, we review
evidence gathered across multiple species demonstrating the links
between
respiration, entrainment of
brain
activity and functional relevance for affecting mood and cognition. We
also discuss further linkages with cardiac rhythms, and the potential
translational implications for biorhythm monitoring and
regulation in neuropsychiatric disorders.
Copyright © 2020 Elsevier Ltd. All rights reserved.
----------------Päivitys 26.2. 2020